Published on September 23rd 2023 | 3 mins , 557 words
Chemistry paper 1 Post Mock 2023
1. (a) Distinguish between ionization energy and electron affinity
Ionization energy refers to the minimum energy required to remove an electron from the outermost energy level of an atom in gaseous state while electron affinity is the energy released when an atom gains an electron in gaseous state
(b) The atomic numbers of A and B are 9 and 17 respectively.Compare the electron affinity of A and B. Explain (2mks)
- A: 2 . 7
- B:2 . 8 . 7
- A has higher electron affinity because it has smaller atomic size with stronger nuclear attraction
2. Natural rubber has the formula


\(\frac{102000}{68}=1500\)
4. Carbon (iv) Oxide was bubbled through concentrated sodium hydroxide solution and no visible change was observed but when bubbled through calcium hydroxide solution for a short time, a white precipitate was formed. Explain
When bubbled through concentrated sodium hydroxide, soluble sodium carbonate is formed but when bubbled through calcium hydroxide for a short time, insoluble calcium hydroxide is formed
5. study the scheme below and answer the questions that follow
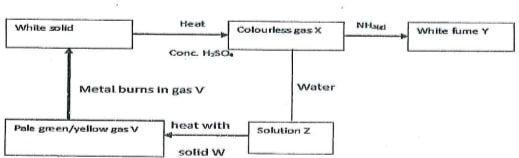
\(HCl_{(g)} + NH_{3(g)} \) \(\rightarrow\) \(NH_4Cl_{(g)}\)
(b) What is the function of solid W in the reaction
It is an oxidising agent.
It oxidises hydrochrogen chloride to chlorine
It removes H from HCl
(c) Identify gas V
Chlorine gas
6. The setup below shows the catalytic oxidation of ammonia gas in the laboratory

Brown fumes evolved
Nitrogen (II) Oxide formed is oxidised by air to nitrogen (IV) Oxide
The platinum catalyst glows - the reaction is highly exothermic
(b) Write a chemical equation for the first reaction taking place in the beaker
\(4NH_{3(g)}\) +\( 5O_{2(g)}\) \(\rightarrow\) \(4NO_{(g)}\) + \(6H_{2}O_{(g)}\)
7. Using an energy cycle diagram, calculate the standard enthalpy of formation of carbon disulphide (3mks)
\(\triangle H_f\) + \(\triangle H_1\) \(\rightarrow\) \(\triangle H_2\) +\(\triangle H_3\)
\(\triangle H_f\) = \(2(-294)\)+\((-393)\) +\(1072\)
=\(-981\)+\(1072\)
= \(+91 kJ/mol\)
8. A bicycle was found to hold a maximum volume of \(990 cm^{3}\) at s.t.p. On one hot sunny day the temperature was \(30^{\circ}C\) and pressure of \(800mmHg\). The rider inflated the tyre. Explain what happened (show your calculation, standard temperature and pressure is \(O^{\circ}C\) and \(760mmHg\) respectively)
By invoking combined gas laws equation, since \(V\) \(\propto\) \(T\) and \(P\) \(\propto\) \(\frac{1}{V}\), it implies that \(\frac{V}{T}=constant\) and \(PV=constant\) thus combining the two we have:
\(\frac{PV}{T}=constant\), that is, \(\frac{P_1V_1}{T_1}= \frac{P_2V_2}{T_2} \)
\(\frac{760\times 990}{273}\)= \(\frac{800\times V_2}{303}\)
\(V_2 =1043.85 cm^{3}\)
Increase in temperature and decrease in pressure increases the volume occupied by a given mass of gas
Tyres bursts.
9. A magnesium ribbon sample was heated in separate volumes of pure oxygen and air.
(a) In which sample was the mass of the product higher? Explain.
Sample heated with air
It combines with both oxygen and nitrogen in air
(b) Write an equation for the reactions in the sample with air
\(3Mg_{(s)}\) +\(N_{2(g)}\) \(\rightarrow\) \(Mg_{3}N_{2(s)}\)
\(2Mg_{(s)}\) + \(O_{2(g)}\) \(\rightarrow\) \(2MgO_{(s)}\)

