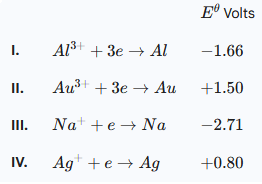Published on January 22nd 2026 | 12 mins , 2332 words
1. Use the section of the periodic table in Table 1 to answer the questions that follow. The letters are not the actual symbols of the elements.
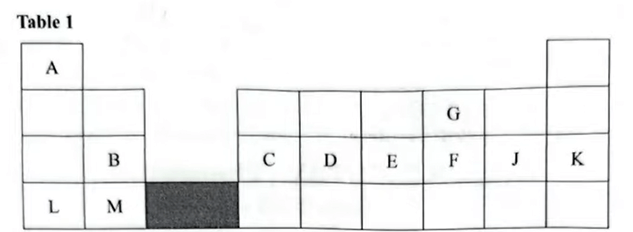
2.8.6
(b) Select two elements which exist as diatomic molecules. (1 mark)
G and J
(c) Select a metal that forms a stable divalent ion with the smallest ionic radius. (1 mark)
B , \(Mg^{2+}\) has more protons, so it pulls the electrons closer
2. 20.0 g of a compound of sodium and oxygen contains 8.2 g of oxygen. Given that the relative formula mass of the compound is 78, determine the:
(a) empirical formula of the compound (O=16.0,Na=23.0). (2 marks)

(b) molecular formula of the compound. (1 mark)
\((NaO)_{n}\) =78
\((23+16)_{n}\)=78
39n=78
n=2
Molecular formula \((NaO)_{2}\) = \(Na_{2}O_{2}\)
3. Carbon (II) oxide is prepared according to the following equation.
\(HCOOH\)→\(CO\)+\(H_{2}O\)
(a) Name the reagent necessary for the reaction to occur. (1 mark)
Concentrated Sulphuric (VI) Acid / H₂SO₄(l)
(b) Explain how: (i) carbon (II) oxide is collected; (1 mark)
Over water method since CO is slightly soluble in water.
- Use of syringe since the density of CO is almost the same as that of air
(ii) inhalation of carbon (II) oxide gas causes poisoning. (1 mark)
CO combines with haemoglobin to form stable compound, carboxyhaemoglobin, preventing transport of oxygen
3. Graham's law of diffusion is used to determine molecular mass of gases.
(a) State Graham's law of diffusion. (1 mark)
- The rate of diffusion of a gas is inversely proportional to the square root of its density, provided the temperature and pressure are constant.
(b) In an experiment, it took 25.0 seconds for ammonia gas to diffuse through a membrane, while it took 36.6 seconds for an equal volume of gas X to diffuse through the same membrane. Calculate the relative molecular mass of gas X. (N = 14.0, H = 1.0)
R.M.M of NH3=14+1×3=17
\(
\frac{T_x}{T_{NH_3}} = \sqrt{\frac{R_{mmx}}{R_{NH_3}}}
\)
\(
\frac{36.6}{25} = \sqrt{\frac{R_{mmx}}{17}}
\)
\(
R_{mmx} = \left(\frac{36.6}{25}\right)^2 \times 17
\)
\(
R_{mmx} = 36.436032
\)
= 36.44
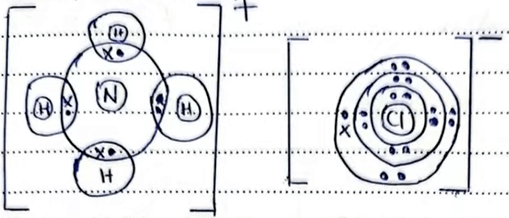
5. (a) Using dots (•) and crosses (×) to represent electrons, draw the structure of ammonium chloride. (H = 1; N = 7; Cl = 17) (2 marks)
(b) Fluorine and iodine are in the same group of the periodic table. Explain why at room temperature, fluorine is a gas while iodine is a solid. (1 mark)
- Iodine has a large atomic / molecular size than fluorine, resulting in more / stronger van der Waals forces than fluorine.
(a) Sea water contains about 3% sodium chloride. Describe how sodium chloride is obtained from the sea water. (2 marks)
- Sea water is channeled into shallow evaporation ponds.
- The water is allowed to evaporate.
- A concentrated brine solution formed and eventually the NaCl crystallizes.
- The crystals collected are purified.
(b) State the reason why iodine is added to common salts for household use. (1 mark)
To prevent iodine deficiency diseases such as goitre.
7. Consider the following standard redox potentials.
(a) Identify the strongest oxidising agent. Give a reason. (1 mark)
\(Au^{3+}\)
- It has the highest positive electrode potential /
- It has the most electropositive electrode potential.
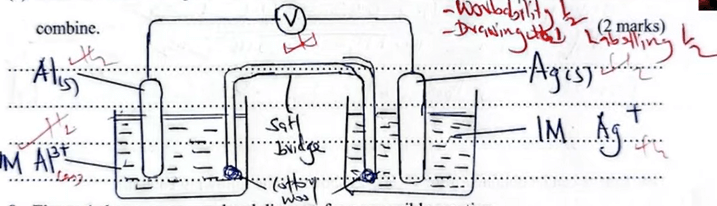
(b) Draw a labelled diagram for an electrochemical cell formed when half-cell I and IV combine
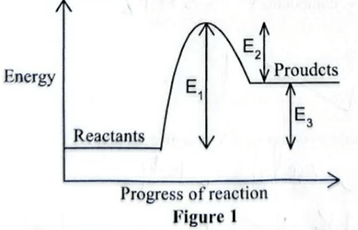
7. Figure 1 shows an energy level diagram for a reversible reaction.
(a) State which of the energies E1, E2 and E3 are affected by a catalyst. Give a reason. (2 marks)
E1,E2 - Catalyst lowers the activation energy.
9. 100 cm3 of 2 M sodium hydroxide was added to 150 cm3 of 3 M sodium hydroxide.
Determine the concentration of the resultant solution. (3 marks)
\(
\text{Moles of } 100~\text{cm}^3 \text{ of } 2\,\text{M NaOH}
= \frac{100}{1000} \times 2
= 0.2~\text{moles}
\)
\(
\text{Moles of } 150~\text{cm}^3 \text{ of } 3\,\text{M NaOH}
= \frac{150}{1000} \times 3
= 0.45~\text{moles}
\)
\(
\text{Total moles} = 0.2 + 0.45 = 0.65~\text{moles}
\)
\(
\text{Total volume} = 100 + 150 = 250~\text{cm}^3
\)
\(
\text{Concentration of the resultant solution}
= \frac{0.65}{250} \times 1000
= 2.6~\text{M}
\)
10. Ethene can be obtained from \(C_{10}H_{22}\) as shown in the following equation.
\(
\mathrm{C_{10}H_{22}} \xrightarrow{\text{heat, pressure}} \mathrm{C_{2}H_{4}} + \text{compound Y}
\)
(a) Name this type of reaction. (1 mark)
Cracking
(b) Write the molecular formula of compound Y and give its name. (1 mark)
\( \mathrm{C_{8}H_{18}} \), Octane
(c) State one industrial use of ethene. (1 mark)
- To make polyethene / Plastic
- used to ripen fruits
- Manufacture of ethanol
- Manufacture of ethane-1,2-diol used as engine coolant
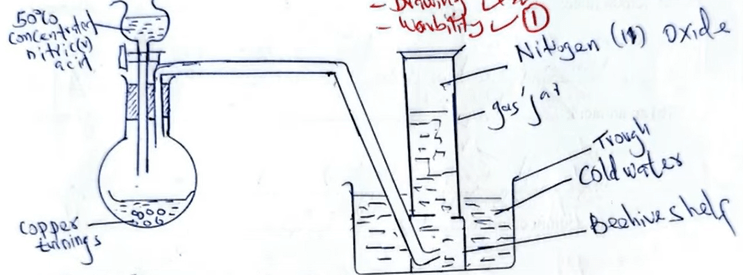
11. Nitrogen (II) oxide can be prepared by reacting copper turnings \( \mathrm{Cu}(s) \) with nitric (V) acid \( \mathrm{HNO_3}(aq) \).
(a) Write an equation for the reaction. (1 mark)
\( 3\mathrm{Cu}(s) \) + \( 8\mathrm{HNO_3}(aq) \) → \( 3\mathrm{Cu(NO_3)_2}(aq) \) + \( 4\mathrm{H_2O}(l) \) + \( 2\mathrm{NO}(g) \)
(b) Draw a set-up of apparatus to show how the gas can be prepared and collected.
12. The following two compounds react to form a polymer.
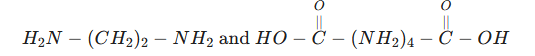
Condensation polymerisation
(b) Draw the general formula of the polymer. (1 mark)
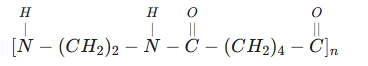
- Non-biodegradable causing environmental pollution.
13. Table 2 shows pH values of solutions of substances P, Q, R, S and T. Use the information to answer the questions that follow
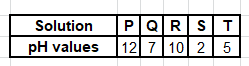
(a) lemon juice: ........................ S (1 mark)
Reason
Citric acid dissociates to give many \(H^{+}\) ions
Lemon juice has Citric acid, make it strongly acidic
(b) an antiacid; P ........ (1 mark)
Reason
— High pH (Alkaline) used to neutralize stomach acidity
(c) aqueous sodium chloride. .................... Q . (1 mark)
Reason .
NaCl dissociates in water to form \(Na^{+}\) and \(Cl^{−}\) . Aqueous sodium chloride is neutral hence it is likely to be Q which has a PH of 7
14 (a) Describe how a dry sample of calcium sulphate can be prepared in the laboratory starting with solid sodium sulphate and solid calcium nitrate. (2 marks)
⇒ Dissolve the solid \(Na_{2}SO_{4}\) and solid \(Ca(NO_{3})_{2}\) separately in water to form aqueous solution.
⇒ Add \(Na_{2}SO_{4}\) solution to \(Ca(NO_{3})_{2}\) solution and stir.
⇒ Filter to obtain \(CaSO_{4}\) as the residue.
− Wash the and dry the residue between two filter papers.
(b) State one use of calcium sulphate. (1 mark)
− Manufacture of Plaster of paris (for making casts, molds, fixing fractured bones)
− Manufacture of cement (as gypsum)
15. The set-up in Figure 2 was used to isolate nitrogen gas from air.
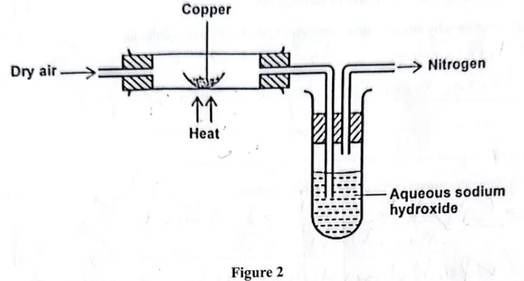
− The brown solid changes to black solid.
(b) Give the role of sodium hydroxide in the boiling tube. (1 mark)
− To remove Carbon (IV) oxide.
(c) Two compounds are formed when copper in the combustion tube is replaced with lithium metal. Give the formula of each compound. (1 mark)
− \(Li_{2}O\)
− \(Li_{3}N\)
16. Beryllium, magnesium and calcium are elements in group II of the periodic table, with atomic numbers 4, 12 and 20 respectively.
(a) Explain why atomic radius increases from beryllium to calcium. (1 mark)
− Increase in the number of occupied energy levels from Be to Ca / down the group.
(b) State the most reactive metal. Give a reason. (1 mark)
⇒ Ca. It has the largest atomic radius − thus outermost electrons are easily lost // ✓ Readily loses electrons due to weak nuclear attraction. // − It is the most electropositive
(c) Beryllium has the highest melting point. Give a reason. (1 mark)
⇒ Beryllium has strongest metallic bond due to the smallest atomic radius // atomic size.
17. 60 g of radioactive astatine-210, \( ^{210}_{85}\text{At}\) reduced to 3.75 g after 33.2 hours.
(a) The radioactive isotope, \(^{210}_{85}\text{At}\) decays by alpha emission. Determine the mass number and number of protons of the nuclide formed.
\(^{210}_{85}\text{At}\) \(\rightarrow\) \(^{206}_{83}\text{X}\) + \(^{4}_{2}\text{He}\)
Mass number (1 mark)
206
Proton number (1 mark)
83
(b) Determine the half-life of astatine (At) (1 mark)
\(
60\,\text{g} \rightarrow 30\,\text{g} \rightarrow 15\,\text{g} \rightarrow 7.5\,\text{g} \rightarrow 3.75\,\text{g}
\)
\(
\text{No. of half-lives } n = 4
\)
\(
T_{\tfrac{1}{2}} = \frac{\text{time elapsed}}{n}
\)
\(
T_{\tfrac{1}{2}} = \frac{33.2}{4}
\)
\(
T_{\tfrac{1}{2}} = 8.3\ \text{hours}
\)
18.
(a) Name two properties of an alloy that are different from those of individual metals. (1 mark)
(i) Harder and stronger. ✓✓ 2
(ii) lower melting points. ✓✓
(ii) More resistant to corrosion/ less reactive.
(b) Complete Table 3 by writing one use of each alloy. (2 marks)
Table 3
Alloy : Brass
Composition : Cu, Zn
Use
- used for screws, nuts and ornaments
- make water taps, door handles, musical instruments
Alloy : Duralumin
Composition : Al, Mg
Use
- used in aircraft, railway trucks, cars
parts, bicycles frames due its low density and high strength
19. The set-up in Figure 3 was used to investigate the electrical conductivity of molten substances.
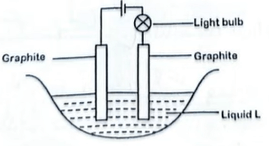
(a) molten sulphur; (1 mark)
Observation
Bulb does not light.
Reason
Sulphur exist as molecules (S8) which do not have mobile ions/delocalized electrons.
(b) molten lead (II) bromide; (1 mark)
Observation
Bulb lights up.
Reason
Molten \(PbBr_{2}\) has mobile ions.
(c) molten sodium metal. (1 mark)
Reason
Bulb lights up.
Explanation
Na has delocalised electrons.
20. Consider the following equilibrium:
\(\text{Cr}_2\text{O}_7^{2-}(\text{aq}) + 2\text{OH}^-(\text{aq}) \rightleftharpoons 2\text{CrO}_4^{2-}(\text{aq}) + \text{H}_2\text{O(l)}\)
(orange) (yellow)
State and explain the observation made when each of the following is added to the equilibrium mixture:
(a) aqueous sodium hydroxide;
Observation
Yellow colour intensifies. (1 mark)
Explanation
NaOH introduces \(OH^{−}\) which increases the concentration of \(OH^{−}\), thus the equilibrium shifts to the right.
(b) dilute hydrochloric acid.
Observation (½ mark)
Orange colour intensifies.
Explanation (1 mark)
HCl reacts with OH⁻, thus reducing OH⁻(aq) concentration thus the equilibrium shifts to the left.
21. Figure 4 shows an energy cycle diagram for caesium chloride.
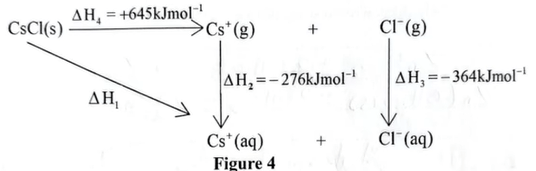
(i) the sign for \(ΔH_{2}\) is negative (exothermic); (1 mark)
\(ΔH_{2}\) is the enthalpy of hydration of gaseous \(Cs^{+}\) ions, which is the energy released when gaseous ions are hydrated.
(ii) the sign of \(ΔH_{4}\) is positive (endothermic). (1 mark)
\(ΔH_{4}\) is Lattice energy of CsCl which the energy required to break bonds holding the solid crystal lattice
(b) Calculate the value of ΔH₁. (1 mark)
ΔH₁ = ΔH₄ + ΔH₂ + ΔH₃ = +645 − 276 − 364 = +645 − 640 = +5 kJ/mol
(a) Zinc oxide is an amphoteric oxide. State what is meant by the term amphoteric. (1 mark)
- A substance (oxide or hydroxide) that exhibit both acidic and basic properties and can react with both acid and base.
(b) When a few drops of aqueous sodium hydroxide are added to an aqueous zinc sulphate, a white precipitate is formed. The white precipitate dissolves when excess sodium hydroxide is added. Write ionic equations for the two reactions. (2 marks)
\(Zn^{2+}_{(aq)}\) + \(2OH^{-}_{(aq)} \rightarrow \) \(Zn(OH)_{2(s)}\)
\(Zn(OH)_{2(s)}\) + \(2OH^{-}_{(aq)} \rightarrow\) \([Zn(OH)_{4}]^{2-}_{(aq)}\)
23 (a) Name the apparatus that can be used to separate a mixture of water and hexane. (1 mark)
Separating funnel
(b) State the use of the following:
(i) desiccator; (1 mark)
Used to keep substances dry / free from moisture
(ii) ion exchange resin. (1 mark)
- Used to remove unwanted ions from a solution and replace them with more desirable ions.
- Used in water softening and water deionization.
24. Oxygen gas can be prepared in the laboratory using aqueous hydrogen peroxide.
(a) State the necessary condition for this reaction. (1 mark) ⇒ Presence of a catalyst, Manganese (IV) oxide / MnO2
\(2H_{2}O_{2(l)}\) \(\rightarrow\) \({MnO_{2}} + 2H_{2}O_{(l)}\) + \(O_{2(g)}\)
(b) Describe how a dry sample of oxygen gas can be collected. (2 marks)
⇒ The oxygen is passed through concentrated sulphuric (VI) acid to be dried and collected by use of a syringe
25. Study the flowchart in figure 5
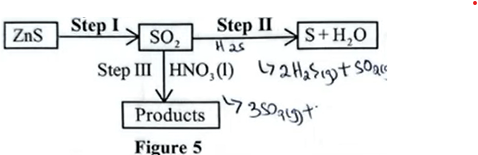
— Heating ✓ + 1/2
— Presence of Air or oxygen. ✓ + 1/2
(b) Name the reducing agent used in step II. (1 mark)
Hydrogen Sulphide ✓ 1 / \(H_{2}S\)
(c) Write a formula of the sulphur compound formed in step III. (1 mark)
\(H_{2}SO_{4}\) ✓ 1
26. The following is a formula of a certain cleansing agent.
\(CH_3(CH_2)_{16}COO^-Na^+\)
(a) Write the formula of the organic compound used to make the cleansing agent. (1 mark)
\(CH_3(CH_2)_{16}COOH\)
(b) Describe how the cleansing agent works during the cleaning process. (2 marks)
— It has a hydrophobic (water-hating), non polar hydrocarbon tail and a hydrophilic (water-loving) polar head. ✓ 1/2
— The hydrophilic heads dissolve in water. ✓ 1/2
— The hydrophobic tails embed themselves in and dissolve in dirt/grease/oil. ✓ 1/2
— This forms tiny micelles with trapped dirt inside. ✓ 1/2
— The polar surface of the micelles allows them to be suspended and carried away by the water, removing the dirt from the surface.
27. Chlorofluoromethane is an example of CFC.
(a) State one industrial use of CFCs. (1 mark)
- As a refrigerants in refrigerators and air
condition ers. - As aerosol propellents in spray cans.
- As a foaming agents in making plastic foams. (Any one)
(b) State one effect of the use of CFCs to the environment. (1 mark)
- They cause the depletion of the ozone layer
in the stratosphere which leads to increased
ultraviolet (UV) radiation reaching the earth’s
surface.